

Corrosion Control Methods
Chemical Treatment
Treatment of corrosive water can be either chemical or physical. Chemicals used in the treatment are either meant to stabilize the water, to form a protective film on the pipe surface, or to kill problematic bacteria.
Stabilizing the water is often the simplest form of corrosion control. When stabilizing corrosive water, alkalinity in the form of lime, soda ash, or caustic soda is usually added to saturate or slightly supersaturate the water with calcium carbonate so that it is stable or slightly scale-forming.
When these chemicals are used to stabilize water, they should be fed after filtration to prevent cementing of the filter sand and may be fed before, during, or after chlorination.
Corrosion inhibitors are used to form thin protective films on pipe walls, which will prevent corrosion. Glassy phosphates such as sodium hexa meta phosphate or tetra sodium pyrophosphate are more widely used, but can increase corrosion rates. Both types of inhibitors require continual application into the water, so dead ends in the distribution system must be flushed at intervals to ensure that fresh water containing the inhibitors reaches these areas as well. A large amount of the inhibitor chemicals ends up forming the film on the pipe walls, but some ends up in the drinking water, though this is not a problem since all inhibitor chemicals are considered safe.
If bacteria are a major component of the corrosion problem, then proper disinfection may be part or all of the answer. Maintaining adequate chlorine residual in the distribution system will kill the bacteria and
Physical Protection
Physical protection against corrosion may be very simple or very complex. On the simple end of the spectrum, corrosion can be prevented by breaking the corrosion cell circuit in some manner.
Metal pipes can be replaced with nonmetals which are non-conductive and will not corrode.
Alternatively, pipes may be lined with portland cement or bituminous or asphaltic compounds to prevent the water from reaching the metal, serving the same purpose.
If galvanic corrosion is a problem, then the two metals can be separated by dielectric couplings. Dielectric couplings are plastic, ceramic, or other non-conductive sections used between the two different types of metal. Since electrons cannot flow through the dielectric coupling, it breaks the circuit and prevents corrosion.
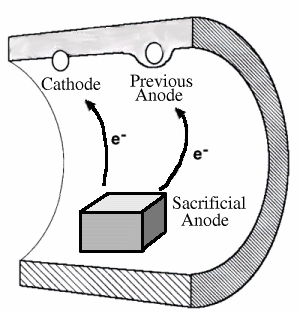
Cathodic Protection using a Sacrificial Anode
More expensive and complicated protection method is cathodic protection, which is the introduction of a different electrical circuit into the pipe. Some cathodic protection systems operate as shown above, by introducing a sacrificial anode into the pipe. A sacrificial anode is a piece of very active metal (usually zinc or magnesium) which is more galvanically active than any other metal in the system. The sacrificial anode will be the only metal corroded, and even previously active anodes on the pipe wall will become cathodes and will thus be protected. Smce the sacrificial anodes slowly corrode away, they must be replaced at intervals, which is the only form of maintenance required on the protection system.
Cathodic protection
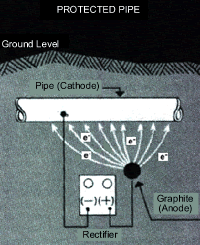
Cathodic Protection
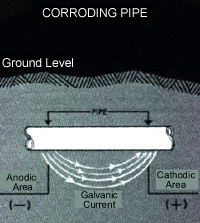
Cathodic Protection
Cathodic protection systems involve the introduction of an external direct current source, known as a rectifier. The rectifier creates a very strong anode since it is constantly producing electrons (an electric current.) This turns the rest of the pipe into a cathode, which prevents any corrosion in the pipe. To complete the circuit, the pipe must be connected back to the rectifier. Direct current cathodic protection systems have been developed which are fully automatic and will compensate for any changes without operator control. However, they also tend to be very expensive to install.
Leave a Reply






 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE