

Coagulation and Flocculation
Types of Suspended Particles in WATER
There are three types of particles which can be found in water in suspension and cause turbidity. In the order from smallest to largest, these particles are chemicals in solution, suspended colloidal solids, and suspended discrete solids.
Some discrete particles of sizes bigger than 0.01 mm such as silt and fine sand causing turbidity would settle out of the water on their own by gravity, if retained for enough time. But other particles would resist settling for days or months due to small particle size and electrical charges between them.
Chemicals in solution have been completely dissolved in the water. They are electrically charged and can interact with the water, so they are completely stable and will never settle out of the water. Chemicals in solution are not visible, either with the naked eye or using a microscope, and are less than one Milli micron (1 mil) in size. An example of a chemical in solution is sugar in water. Colloids, also known as non settleable solids, do not dissolve in water although they are electrically charged. Still, the particles are so small that they will not settle out of the water by gravity even after several years of retention and they cannot be removed by filtration alone. Colloidal solids range between 0.001 and 0.5 micron (11) in size and can be seen only with a high-powered microscope. Examples include bacteria, fine clays, and silts. Colloidal solids often cause colored water, such as the “tea color” of swamp water.
Suspended settle able solids will settle out of water over time, though this may be so slow that it is impractical to merely allow the particles to settle out in a water treatment plant. The particles are more than 0.001 mm (1 /-1) in size and can be seen with a microscope or, sometimes, with the naked eye. Examples of suspended solids include sand and heavy silts.
PURPOSE OF COAGULATION AND FLOCCULATION
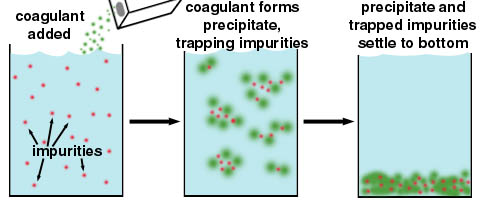
The primary purpose of the coagulation and flocculation is to destabilize the charged colloidal particles in water and make them to settle so as to remove turbidity from the water. In addition to removing turbidity from the water, coagulation and flocculation process removes many bacteria which are suspended in the water and can be used to remove color from the water.
- Characteristics of Colloids
The principal phenomena that control the behaviour of the colloids are zeta potential (electrostatic force), Vander Walls forces and Brownian motion. The amount of coagulant to be added to the water will depend on the zeta potential, a measurement of the magnitude of electrical charge surrounding the colloidal particles. The zeta potential is the amount of repulsive force or electric charge, which keeps the particles in the water. If the zeta potential is large, then more coagulants will be needed.
Van der Waal’s forces refer to the tendency of particles in nature to attract each other weakly if they have no charge.
Once the particles in water are not repelling each other, van der Waal’ s forces make the particles drift toward each other and join together into a group.
Colloids have a sufficiently small mass that collusion with molecular size particles in water will cause constant movement of the colloids. The phenomenon of constant random movement of colloids is known as Brownian motion. The combination of positive and negative charge, results in a neutral, or lack of charge. As a result, the particles no longer repel each other. When enough particles have joined together, they become floc and will settle out of the water.
- Chemistry of Coagulation and Flocculation
The chemistry of coagulation and flocculation is primarily based on electricity. Electricity is the behavior of negatively and positively charged particles due to their attraction and repulsion. Like charges (two negatively charged particles or two positively charged particles) repel each other while opposite charges (a positively charged particle and a negatively charged particle) attract. Most particles dissolved in water have negative charge, so they tend to repel each other. As a result, they stay dispersed and dissolved or Coagulation process colloidal in the water. Addition of positively charged particles in the coagulation process is aimed to destabilizing the colloids. So treatment involving coagulation and flocculation is typical of surface water.
The purpose of addition of coagulant chemicals is to neutralize the negative charges on the colloidal particles to prevent those particles from repelling each other. Coagulants due to their positive charge attract negatively charged particles in the water.
Coagulation is a unit process of addition of coagulant chemicals to water and rapid mixing so as to neutralize the electrical charges of the colloidal particles in the water, and allow them to come closer and form fine clumps or micro flocs.
5 Responses to “Coagulation and Flocculation”
Leave a Reply






 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE
Great article
I have actually been attempting to get in touch with you however I can’t seem to find the right email address ….
Our customer is a factory producing semi cooked potato .The waste of the productions is water mixed with starch and oil.our customer interested to purchase a system to remove the oil and the starch in waste water.
as an up coming engineer ave learned a lot how i wish to work with you
Well summarized and am get well understanding all about coagulation can I say thanks