ORP (Oxidation Reduction Potential)
ORP Applications
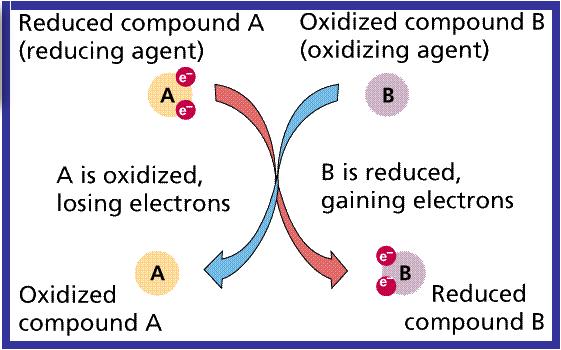
Oxidation Reduction Potential or Redox is the activity or strength of oxidizers and reducers in relation to their concentration. Oxidizers accept electrons, reducers lose electrons.
Examples of oxidizers are: chlorine, hydrogen peroxide, bromine, ozone, and chlorine...














 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE