

Corrosion in Water Treatment
Corrosion in Water Treatment Equipment
Corrosion is the rusting of metal in pipes or tanks due to the corrosive action of either water or soil. Scales are deposits caused by the deposition of calcium carbonate on the inner surface of the water treatment equipment pipes or tanks. Stable water is the water, which tends to be neither corrosive nor scale-forming. Corrosive, also known as aggressive or unstable, water will tend to corrode (rust) metal in the pipes or tanks it passes through. Scale-forming water will tend to deposit calcium carbonate scale on the inner surfaces of these pipes or tanks.
Corrosive and scale-forming waters are at the opposite ends of a spectrum. A variety of water characteristics combine to influence water’s location along this spectrum. The goal of the corrosion control in water treatment is to render the water stable and will neither corrode pipes nor form scale.
Primary Water Characteristics
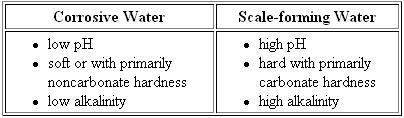
The chemical characteristics such as alkalinity, hardness and pH of the water flowing through a pipe will influence corrosive reaction. The below table summarizes characteristics of corrosive water and of scale-forming hard water.
Chemical characteristics of corrosive and scale forming water
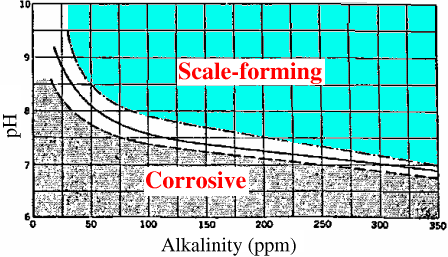
The graph shown below is known as the Baylis Curve. It shows the relationship between pH, alkalinity, and water stability. Water above the lines is scale-(orming while water below the lines is corrosive. Stable water is found in the white area between the lines.
Secondary Water Characteristics
Other chemicals and compounds such as oxygen, carbon dioxide, and dissolved solids found in water also influence the corrosion process. Oxygen reacts with hydrogen gas at the cathode, causing depolarization and speeding up the corrosion. As a result, water with a high D.O. (dissolved oxygen) will tend to be corrosive. Other oxidizing agents such as nitrate and chlorine can perform the same function, although they are less common. Carbon dioxide in water also tends to cause corrosion. The carbon dioxide gas can combine with water to form carbonic acid, which lowers the pH of the water and a low pH promotes corrosion. Dissolved solids are typically present in water as ions. These ions increase the electrical conductivity of the water, making the electrolyte more effective. Thus, they will increase the rate of corrosion.
Physical Water Characteristics
In addition to the chemical properties of water, physical characteristics such as temperature and velocity of flow will influence corrosion. Temperature speeds up the rate of corrosion just as it does most other reactions. However, the effect of temperature on corrosion can be more complex. A high water temperature reduces the solubility of calcium carbonate in water, which promotes scale formation and slows corrosion. Temperature also alters the form of corrosion. Pits and tubercles tend to form in cold water while hot water promotes uniform corrosion. Uniform corrosion spreading across the entire surface of a pipe is far less problematic than tuberculation, so high temperatures can actually seem to slow the corrosive process.
The influence of flow velocity on corrosion is also rather complex. Moderate flow rates are the most beneficial since they promote the formation of scale without breaking loose tubercles. At low flow velocities, corrosion is increased and tends to be in the form of tuberculation due to the prevalence of oxygen concentration cell corrosion. At very high flow velocities, abrasion of the water against the pipe tends to wear the pipe away in a very different form of corrosion. High flow velocities also remove protective scale and tubercles and increase the contact of the pipe with oxygen, all of which will increase the rate of corrosion.
Effects of Bacteria on corrosion
Bacteria can both cause and accelerate the rate of corrosion. In general, bacterial colonies on pipe walls accelerate corrosion below them due to oxygen cell concentration, causing increased pitting and tuberculation. Like humans, some bacteria produce carbon dioxide, which can combine with water to become carbonic acid and accelerate corrosion. Iron bacteria also block the deposition of calcium carbonate scale on the pipe walls. Sulfate-reducing bacteria in addition to causing corrosion produce a distinctive rotten egg smell.
One Response to “Corrosion in Water Treatment”
Leave a Reply







 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE
Corrosion cause in water treatment post is very useful.