

REMOVAL OF IRON AND MANGANESE
TREATMENTS OF WATER
These are, generally, present in very small quantities in water and that too in the absence of dissolved oxygen. When water containing dissolved iron and manganese comes in contact with oxygen/ air, they are converted into red and black precipitates respectively of Fe(OH3), and Mn(OH)2. The presence of iron and manganese in water is highly undesirable, particularly for laundries, dyeing and tanning industries. Moreover, they impart metallic taste to water. The presence of iron and manganese in the water up to 03 irglittre is tolerable and no treatment is needed to remove them. But if their content exceed 0.3 mg/litre, their removal becomes necessary.
They can be removed, conveniently, by spraying water in air or blowing air in water or by chlorination, when insoluble precipitate of Fe(OH)3 and Mn(OH)2 is produced. This precipitate can then be removed by sedimentation and filtration.
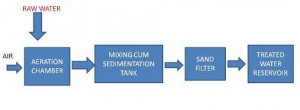
The raw water enters the aeration chamber, in which is blown in. After the aeration, water enters the sedimentation or setting tank, where most hydroxides of Iron and Manganese ( Fe and Mn ) settle down.
The water is then allowed to pass through a sand bed. Filtered water is stored in reservoir for onward distribution.
Leave a Reply






 LIKE TO GET UPDATES
LIKE TO GET UPDATES  TO GET EXPERT GUIDE
TO GET EXPERT GUIDE